Tapers are used in modular devices, like shoulder and hip prothesis. This method assesses the axial locking forces of these tapers. Full description of ASTM F2009 can be found at www.astm.org. The ADMET eXpert 2600 will perform the Constant Rate Assembly and Disassembly Procedure and provide the appropriate fixtures needed to secure the tapers during testing.
ASTM F2009-Testing Systems for Axial Disassembly Force of Taper
Topics: ASTM Tests, Biomedical, implants, Testing Tips
ASTM F2346 Static and Dynamic Spinal Artificial Discs Testing System
These tests allow the user to compare the mechanical properties of artificial discs intended for different locations (Cervical, Thoracic, and Lumbar). This is a summary of the specification ASTM F2346, please read the full specification for details.
Topics: ASTM Tests, Biomedical, implants
ASTM F1798 describes the procedure for testing subassemblies and interconnection mechanisms to determine the fatigue strength and resistance to loosening during arthrodesis.(fusion)
Topics: ASTM Tests, Biomedical, implants, Biaxial
Equipment Required for Testing Coated Fabrics and Textiles ASTM D751
Specification ASTM D751 includes test methods for covering a wide range of mechanical properties of coated fabrics. A coated fabric is a material that has at least one layer of a textile and one layer of a polymeric substance. Examples would be tarps, outdoor clothing, and rain wear. Some of these tests require specialized equipment (hydrostatic resistance, blocking, wicking, impact, etc.), but most of these tests can be performed using a Universal Testing Machine. These tests include:
Topics: ASTM Tests, Textile, rubber, Plastic, universal testing machine, Adhesive, Packaging
Testing System for Bioabsorbable Plates and Screws - ASTM F2502
ASTM F2502 is a standard for testing Bioabsorbable Plates and Screws for Internal Fixation Implants.
Topics: ASTM Tests, Biomedical, Torsion, Bend Testing, implants
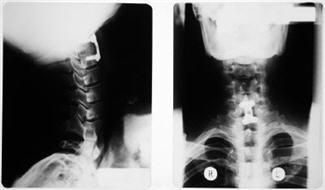
Spinal Implants and Intervertebral Fusion Devices are tested according to several ASTM standards. ASTM F2267 is intended to provide a basis for the mechanical comparison among past, present and future non-biologic intervertebral body fusion devices. These, sometimes hollow, devices are inserted to promote arthrodesis(fusion) at a given spinal motion segment. Construct are governed by ASTM F1717. The mechanical tests are conducted using simplified loading schemes and do not attempt to mimic the complex loads of the spine. An ADMET eXpert 2600 series Testing System equipped with MTESTQuattro will perform the tests required for this specification. An simple outline of the test procedures is as follows:
Topics: ASTM Tests, Biomedical, implants
Testing System for ASTM F2077-03 Intervertebral Body Fusion Device
ASTM F2077-03 is a standard test method for static and dynamic testing of intervertebral body fusion device assemblies. This test method will provide information to compare the mechanical characteristics of different assemblies for different spinal locations and various methods of inserting these assemblies. This standard focuses on all aspects of the spine; cervical, thoracic, and lumber.
Topics: ASTM Tests, Biomedical, implants
How to Perform an ASTM D790 Plastic Flexural 3 Point Bend Test
Along with ASTM D638, D790 is one of the most commonly used specifications in the plastics industry. This test measures the flexural strength and flexural modulus of reinforced and unreinforced plastics. These calculations allow you to choose materials that do not bend when supporting the loads you require for your application. These calculations relate to the stiffness of your material. The test uses a universal testing machine and a three point bend fixture to bend plastic test bars to acquire the data needed to make the calculations. The calculations and set-up for D790 are more complex and time consuming compared to other tests so please read the entire specification from ASTM before running the test. ISO-180 is similar in concept to this test although the specimen shapes and some other details are slightly different. This is a simple summary to help you determine if this specification is right for you and also to call out what testing equipment is needed.
Topics: ASTM Tests, universal testing machine, Tensile Test, Bend Testing
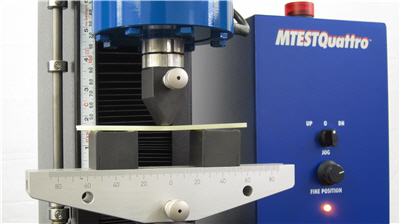
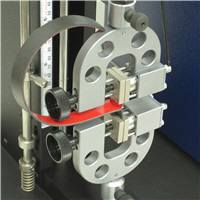
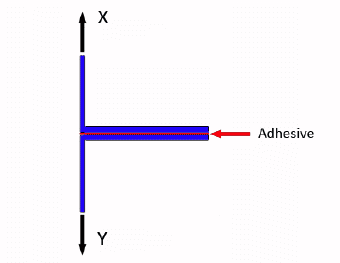
How to Perform an Adhesive Strength T-Peel Test - ASTM D1876
Topics: ASTM Tests, Adhesive
ASTM F2256 describes in detail how to prepare and test tissue adhesives. This standard describes the methods used to compare and characterize different types of adhesives and for manufacturing quality control of the tissue adhesive based medical devices.
Topics: Biomedical, Adhesive, Tissue